How many moles are represented by 11.5 g of c2h5oh – Exploring the relationship between mass and moles, this discussion investigates how many moles are represented by 11.5 g of ethanol (C2H5OH). Delving into the concept of molar mass and its significance, we will derive a formula to calculate moles from mass and apply it to determine the number of moles in the given sample.
Concept of Molar Mass
Molar mass is the mass of one mole of a substance. It is a fundamental property of a substance and is expressed in grams per mole (g/mol). Molar mass plays a crucial role in stoichiometry, which is the study of the quantitative relationships between reactants and products in chemical reactions.
The molar mass of a compound is directly proportional to the number of moles present in a given mass of that compound. This relationship is expressed by the following equation:
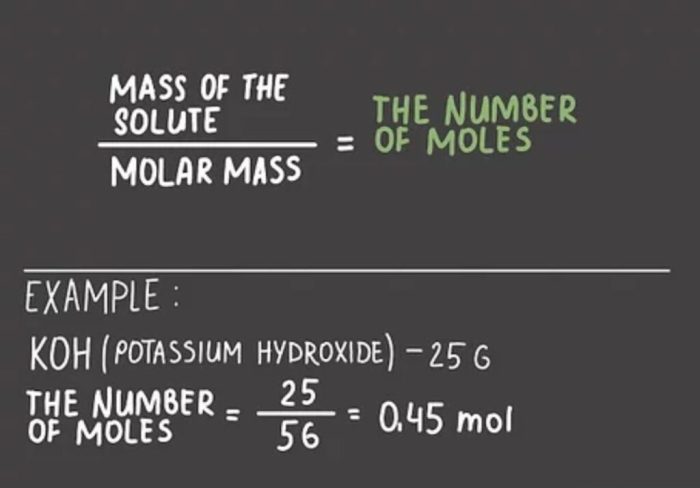
moles = mass / molar mass
Calculating Moles from Mass: How Many Moles Are Represented By 11.5 G Of C2h5oh
To calculate the number of moles in a given mass of a substance, we use the formula:
moles = mass / molar mass
For example, to calculate the number of moles in 11.5 g of ethanol (C2H5OH), we first need to determine its molar mass.
Molar Mass of C2H5OH
The molar mass of C2H5OH can be calculated using the atomic masses of its constituent elements:
molar mass of C2H5OH = (2 x atomic mass of C) + (6 x atomic mass of H) + (1 x atomic mass of O)
Using the atomic masses from the periodic table, we get:
molar mass of C2H5OH = (2 x 12.01 g/mol) + (6 x 1.01 g/mol) + (1 x 16.00 g/mol) = 46.07 g/mol
Units and Dimensional Analysis
It is important to use the correct units in the calculation. In this case, the mass is given in grams and the molar mass is in grams per mole. To ensure that the units cancel out appropriately, we can use dimensional analysis:
moles = 11.5 g C2H5OH x (1 mol C2H5OH / 46.07 g C2H5OH) = 0.250 mol C2H5OH
Table of Values
| Parameter | Value | Units |
|---|---|---|
| Mass of C2H5OH | 11.5 | g |
| Molar mass of C2H5OH | 46.07 | g/mol |
| Number of moles | 0.250 | mol |
FAQ Summary
What is the significance of molar mass?
Molar mass is a crucial concept in chemistry as it represents the mass of one mole of a substance. It allows us to convert between the mass and the number of moles of a substance, which is essential for various stoichiometric calculations.
Can this formula be applied to other substances besides ethanol?
Yes, the formula moles = mass / molar mass is a general formula that can be applied to any substance. As long as we know the mass of the substance and its molar mass, we can calculate the number of moles.